Antes de irte...
¡Obtén un 20% de descuento en tu primer pedido!
20% de descuento
Introduce el código que aparece a continuación al finalizar la compra para obtener un 20 % de descuento en tu primer pedido.
No se pudo cargar la disponibilidad de recogida
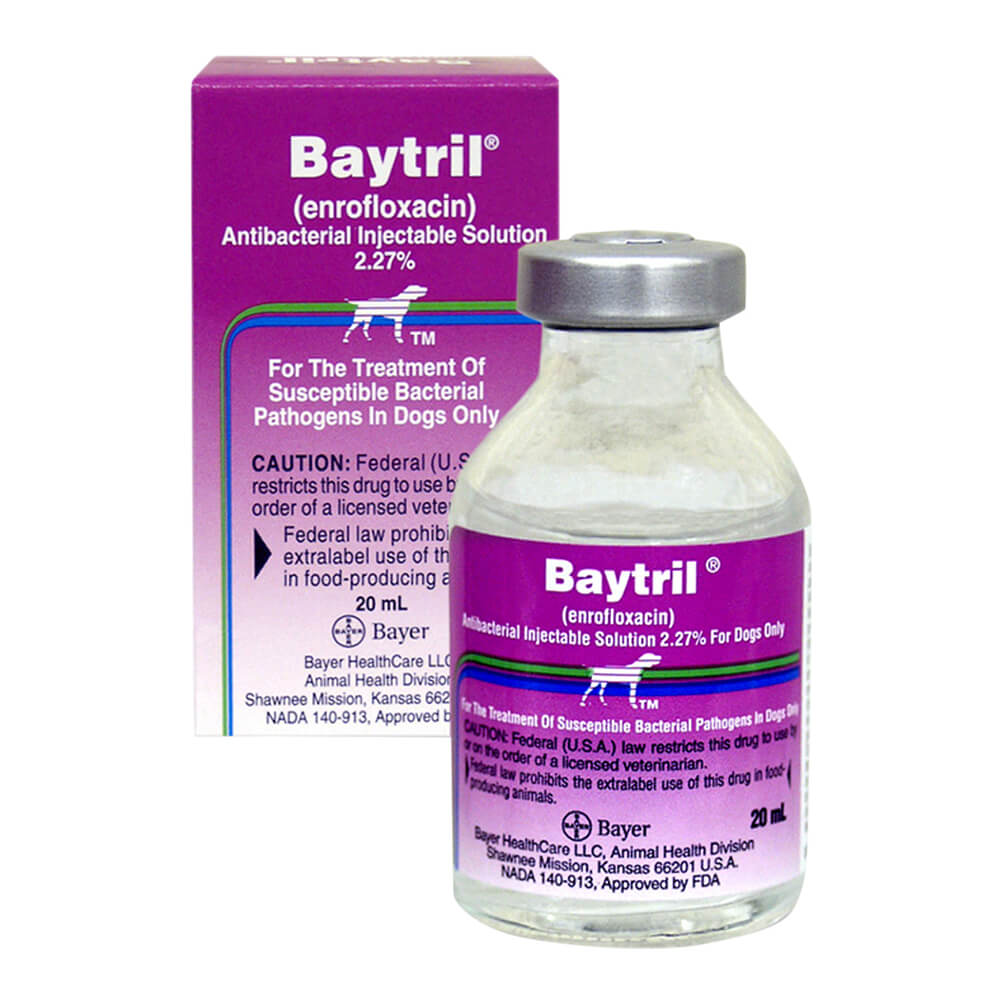
Baytril 2.27% Injectable Solution Baytril (enrofloxacin) Injectable Solution 2.27% is a broad-spectrum fluoroquinolone indicated for the management of diseases in dogs associated with bacteria susceptible to enrofloxacin. It has activity against both gram-negative and gram-postive bacteria, including those causing dermal, urinary and respiratory tract infections. Baytril Injectable Solution 2.27% is approved for use in dogs only. With more than 20 years of performance, Baytril Injectable Solution 2.27% kills a broad range of disease-causing bacteria, right at the site of infection. The concentration-dependent and bactricidal soution is packaged in one 20mL vial box. Prescription items are NON-RETURNABLE and NON-REFUNDABLE. Download Baytril Information Sheet - CAUTIONS/WARNINGS:CAUTION: Federal (U.S.A.) law restricts this drug to use by or on the order of a licensed veterinarian. CONTRAINDICATIONS: Enrofloxacin is contraindicated in dogs known to be hypersensitive to quinolones. Based on the studies discussed under the section on Animal Safety Summary, the use of enrofloxacin is contraindicated in small and medium breeds of dogs during the rapid growth phase (between 2 and 8 months of age). The safe use of enrofloxacin has not been established in large and giant breeds during the rapid growth phase. Large breeds may be in this phase for up to one year of age and the giant breeds for up to 18 months. In clinical field trials utilizing a daily oral dose of 5.0 mg/kg, there were no reports of lameness or joint problems in any breed. However, controlled studies with histological examination of the articular cartilage have not been conducted in the large or giant breeds. WARNINGS: For use in animals only. The use of this product in cats may result in Retinal Toxicity. Keep out of reach of children. Avoid contact with eyes. In case of contact, immediately flush eyes with copious amounts of water for 15 minutes. In case of dermal contact, wash skin with soap and water. Consult a physician if irritation persists following ocular or dermal exposure. Individuals with a history of hypersensitivity to quinolones should avoid this product. In humans, there is a risk of user photosensitization within a few hours after excessive exposure to quinolones. If excessive accidental exposure occurs, avoid direct sunlight. PRECAUTION: Quinolone-class drugs should be used with caution in animals with known or suspected Central Nervous System (CNS) disorders. In such animals, quinolones have, in rare instances, been associated with CNS stimulation which may lead to convulsive seizures. Quinolone-class drugs have been associated with cartilage erosions in weight-bearing joints and other forms of arthropathy in immature animals of various species. The use of fluoroquinolones in cats has been reported to adversely affect the retina. Such products should be used with caution in cats. ANIMAL SAFETY SUMMARY: Adult dogs receiving enrofloxacin orally at a daily dosage rate 52 mg/kg for 13 weeks had only isolated incidences of vomition and inappetence. Adult dogs receiving the tablet formulation for 30 consecutive days at a daily treatment of 25 mg/kg did not exhibit significant clinical signs nor were there effects upon the clinical chemistry, hematological or histological parameters. Daily doses of 125 mg/kg for up to 11 days induced vomition, inappetence, depression, difficult locomotion and death while adult dogs receiving 50 mg/kg/day for 14 days had clinical signs of vomition and inappetence. Adult dogs dosed intramuscularly for three treatments at 12.5 mg/kg followed by 57 oral treatments at 12.5 mg/kg, all at 12 hour intervals, did not exhibit either significant clinical signs or effects upon the clinical chemistry, hematological or histological parameters. Oral treatment of 15 to 28 week old growing puppies with daily dosage rates of 25 mg/kg has induced abnormal carriage of the carpal joint and weakness in the hindquarters. Significant improvement of clinical signs is observed following drug withdrawal. Microscopic studies have identified lesions of the articular cartilage following 30 day treatments at either 5, 15 or 25 mg/kg in this age group. Clinical signs of difficult ambulation or associated cartilage lesions have not been observed in 29 to 34 week old puppies following daily treatments of 25 mg/kg for 30 consecutive days nor in 2 week old puppies with the same treatment schedule. Tests indicated no effect on circulating microfilariae or adult heartworms (Dirofilaria immitis) when dogs were treated at a daily dosage rate of 15 mg/kg for 30 days. No effect on cholinesterase values was observed. No adverse effects were observed on reproductive parameters when male dogs received 10 consecutive daily treatments of 15 mg/kg/day at 3 intervals (90, 45 and 14 days) prior to breeding or when female dogs received 10 consecutive daily treatments of 15 mg/kg/day at 4 intervals; between 30 and 0 days prior to breeding, early pregnancy (between 10th & 30th days), late pregnancy (between 40th & 60th days), and during lactation (the first 28 days). DRUG INTERACTIONS: Concomitant therapy with other drugs that are metabolized in the liver may reduce the clearance rates of the quinolone and the other drug. Enrofloxacin has been administered to dogs at a daily dosage rate of 10 mg/kg concurrently with a wide variety of other health products including anthelmintics (praziquantel, febantel), insecticides (pyrethrins), heartworm preventatives (diethylcarbamazine) and other antibiotics (ampicillin, gentamicin sulfate, penicillin). No incompatibilities with other drugs are known at this time. - DIRECTIONS:DOSAGE AND ADMINISTRATION: Baytril Injectable Solution may be used as the initial dose at 2.5 mg/kg. It should be administered intramuscularly (IM) as a single dose, followed by initiation of Baytril Tablet therapy. Baytril Injectable Solution may be administered as follows: Weight of Animal Baytril Injectable Solution* 2.5 mg/kg 20 lbs (9.1 kg) 1.00 ml 60 lb (27.2 kg) 3.00 ml *The initial Baytril Injectable administration should be followed 12 hours later by initiation of Baytril Tablet therapy. The lower limit of the dose range was based on efficacy studies in dogs where enrofloxacin was administered at 2.5 mg/kg twice daily. Target animal safety and toxicology studies were used to establish the upper limit of the dose range and treatment duration. - INGREDIENTS:Active Ingredients: Enrofloxacin - HEIGHT(INCHES): 1.000 - WIDTH(INCHES):1.000 - LENGTH(INCHES):1.000
Puedes devolver la mayoría de los artículos nuevos y sin abrir en un plazo de 30 días a partir de la fecha de entrega para obtener un reembolso completo. También cubriremos los gastos de envío de la devolución si esta se debe a un error nuestro (recibiste un artículo incorrecto o defectuoso, etc.).
Debería recibir su reembolso en un plazo de cuatro semanas a partir de la fecha en que entregue el paquete a la empresa de mensajería, aunque en muchos casos lo recibirá antes. Este plazo incluye el tiempo de tránsito para que recibamos su devolución (de 5 a 10 días hábiles), el tiempo que tardamos en procesarla una vez recibida (de 3 a 5 días hábiles) y el tiempo que tarda su banco en procesar nuestra solicitud de reembolso (de 5 a 10 días hábiles).
Si necesita devolver un artículo, inicie sesión en su cuenta, consulte el pedido mediante el enlace «Pedidos Completados» en el menú «Mi Cuenta» y haga clic en el botón «Devolver artículo(s)». Le notificaremos el reembolso por correo electrónico una vez que hayamos recibido y procesado el artículo devuelto.
Realizamos envíos a prácticamente cualquier dirección del mundo. Tenga en cuenta que existen restricciones para algunos productos y que algunos no se pueden enviar a destinos internacionales.
Al realizar un pedido, calcularemos las fechas de envío y entrega según la disponibilidad de los artículos y la opción de envío que elija. Dependiendo del proveedor de envío, las fechas estimadas de entrega podrían aparecer en la página de cotización de envío.
Tenga en cuenta que los gastos de envío de muchos de nuestros artículos se calculan según el peso. El peso de cada artículo se indica en su página de detalles. Para ajustarnos a las políticas de las empresas de transporte, todos los pesos se redondearán a la libra entera superior.
