Antes de irte...
¡Obtén un 20% de descuento en tu primer pedido!
20% de descuento
Introduce el código que aparece a continuación al finalizar la compra para obtener un 20 % de descuento en tu primer pedido.
No se pudo cargar la disponibilidad de recogida
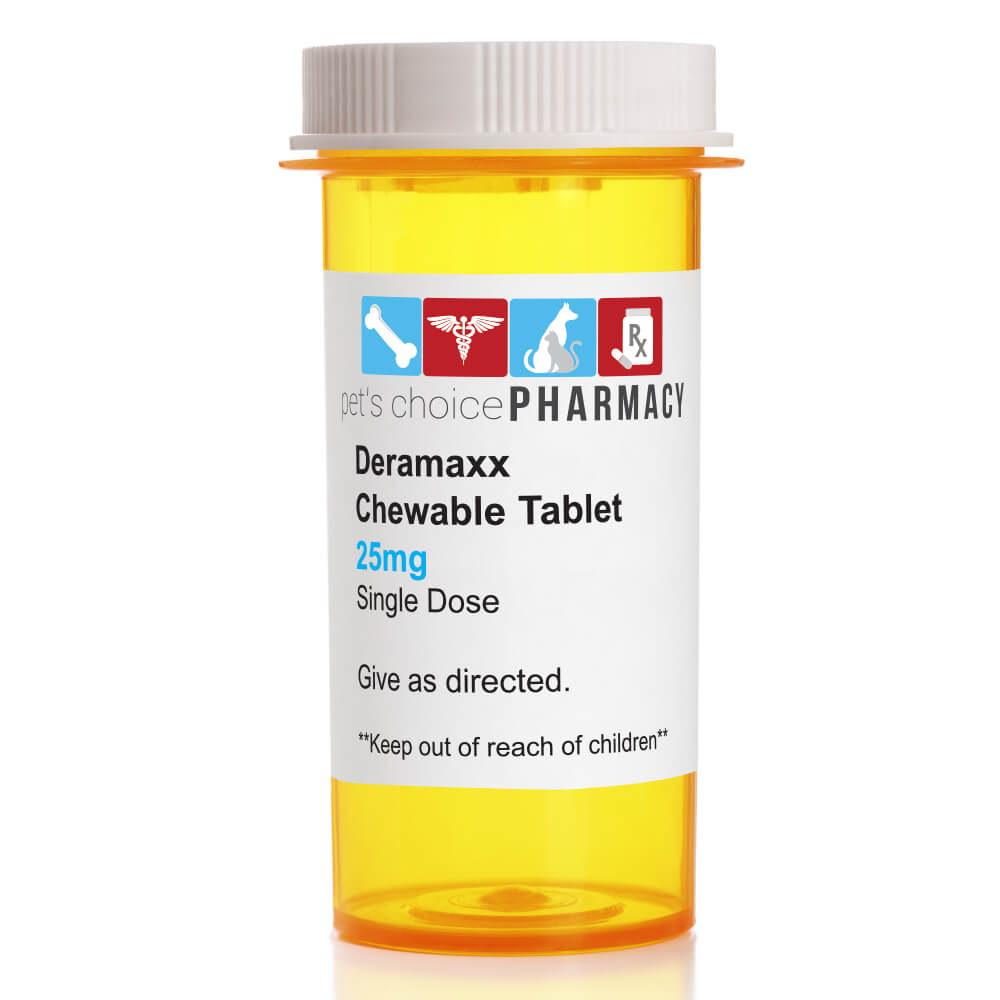
Deramaxx DERAMAXX (deracoxib) is a non-narcotic, non-steroidal anti-inflammatory drug of the coxib class. DERAMAXX tablets are round, biconvex, chewable tablets that contain deracoxib formulated with beefy flavoring. The molecular weight of deracoxib is 397.38. The empirical formula is C17-H14-F3-N3-O3-S. Deracoxib is 4-[3-(difluoromethyl)-5-(3-fluoro-4-methoxyphenyl)-1H-pyrazole-1-yl] benzenesulfonamide, and can be termed a diaryl substituted pyrazole. Non-linear elimination kinetics are exhibited at doses above 8 mg/kg/day, at which competitive inhibition of constitutive COX-1 may occur. Deracoxib is not excreted as parent drug in the urine. The major route of elimination of deracoxib is by hepatic biotransformation producing four major metabolites, two of which are characterized as products of oxidation and o-demethylation. The majority of deracoxib is excreted in feces as parent drug or metabolite. Large intersubject variability was observed in drug metabolite profiles of urine and feces. No statistically significant differences between genders were observed. All prescription items are Non-Refundable and Non-Returnable. - CAUTIONS/WARNINGS:Warnings: Not for use in humans. Keep this and all medications out of reach of children. Consult a physician in case of accidental ingestion by humans. For use in dogs only. All dogs should undergo a thorough history and physical examination before the initiation of NSAID therapy. Appropriate laboratory tests to establish hematological and serum biochemical baseline data prior to, and periodically during, administration of any NSAID is recommended. Owners should be advised to observe for signs of potential drug toxicity (see Adverse Reactions, Animal Safety and Post-Approval Experience) and be given an "Information for Dog Owners" Sheet. Sensitivity to drug-associated adverse events varies with the individual patient. As a class, NSAIDs may be associated with gastrointestinal, renal and hepatic toxicity. Patients at greatest risk for NSAID toxicity are those that are dehydrated, on concomitant diuretic therapy, or those with existing renal, cardiovascular, and/or hepatic dysfunction. Since many NSAIDs possess the potential to produce gastrointestinal ulceration and/or perforation, concomitant use of DERAMAXX tablets with other anti-inflammatory drugs, such as NSAIDs or corticosteroids, should be avoided. Precautions: Plasma levels of deracoxib may increase in a greater than dose-proportional fashion above 8 mg/kg/day. DERAMAXX tablets have been safely used during field studies in conjunction with other common medications, including heartworm preventatives, anthelmintics, anesthetics, pre-anesthetic medications, and antibiotics. If additional pain medication is needed after a daily dose of DERAMAXX tablets, a non-NSAID/non-corticosteroid class of analgesic may be necessary. It is not known whether dogs with a history of hypersensitivity to sulfonamide drugs will exhibit hypersensitivity to DERAMAXX tablets. The safe use of DERAMAXX tablets in dogs younger than 4 months of age, dogs used for breeding, or in pregnant or lactating dogs has not been evaluated. NSAIDs may inhibit the prostaglandins which maintain normal homeostatic function. Such anti-prostaglandin effects may result in clinically significant disease in patients with underlying or pre-existing disease that has not been previously diagnosed. Appropriate monitoring procedures should be employed during all surgical procedures. The use of parenteral fluids during surgery should be considered to decrease potential renal complications when using NSAIDs perioperatively. Concurrent administration of potentially nephrotoxic drugs should be carefully approached. The use of concomitantly protein-bound drugs with DERAMAXX tablets has not been studied in dogs. Commonly used protein-bound drugs include cardiac, anticonvulsant and behavioral medications. The influence of concomitant drugs that may inhibit metabolism of DERAMAXX tablets has not been evaluated. Drug compatibility should be monitored in patients requiring adjunctive therapy. Consider appropriate washout times when switching from one NSAID to another or when switching from corticosteroid use to NSAID use. - DIRECTIONS:Osteoarthritis Pain and Inflammation:0.45 - 0.91 mg/lb/day 1 to 2 mg/kg/day) as a single daily dose, as needed. Postoperative Orthopedic Pain and Inflammation: DERAMAXX Chewable Tablets are indicated for the control of postoperative pain and inflammation associated with orthopedic surgery in dogs = 4 lbs (1.8 kg). Dosage and Administration: Postoperative Orthopedic Pain and Inflammation: 1.4-1.8 mg/lb/day (3 to 4 mg/kg/day) as a single daily dose, as needed, not to exceed 7 days of administration. Always provide "Information for Dog Owners" Sheet with prescription. Since DERAMAXX tablet bioavailability is greatest when taken with food, postprandial administration is preferable. However, DERAMAXX tablets have been shown to be effective under both fed and fasted conditions; therefore, they may be administered in the fasted state if necessary. For postoperative orthopedic pain, administer DERAMAXX tablets prior to the procedure. Tablets are scored and dosage should be calculated in half-tablet increments. In clinical practice it is recommended to adjust the individual patient dose while continuing to monitor the dog's status until a minimum effective dose has been reached. Contraindications: Dogs with known hypersensitivity to DERAMAXX or other NSAIDs should not receive DERAMAXX tablets. Storage Conditions: DERAMAXX tablets should be stored at room temperature between 59° and 86°F (15-30°C). Keep this and all medications out of reach of children. - STORAGE/DISPOSAL REQ:Store at 59-86 degrees F - HEIGHT(INCHES): 1.000 - WIDTH(INCHES):1.000 - LENGTH(INCHES):1.000
Puedes devolver la mayoría de los artículos nuevos y sin abrir en un plazo de 30 días a partir de la fecha de entrega para obtener un reembolso completo. También cubriremos los gastos de envío de la devolución si esta se debe a un error nuestro (recibiste un artículo incorrecto o defectuoso, etc.).
Debería recibir su reembolso en un plazo de cuatro semanas a partir de la fecha en que entregue el paquete a la empresa de mensajería, aunque en muchos casos lo recibirá antes. Este plazo incluye el tiempo de tránsito para que recibamos su devolución (de 5 a 10 días hábiles), el tiempo que tardamos en procesarla una vez recibida (de 3 a 5 días hábiles) y el tiempo que tarda su banco en procesar nuestra solicitud de reembolso (de 5 a 10 días hábiles).
Si necesita devolver un artículo, inicie sesión en su cuenta, consulte el pedido mediante el enlace «Pedidos Completados» en el menú «Mi Cuenta» y haga clic en el botón «Devolver artículo(s)». Le notificaremos el reembolso por correo electrónico una vez que hayamos recibido y procesado el artículo devuelto.
Realizamos envíos a prácticamente cualquier dirección del mundo. Tenga en cuenta que existen restricciones para algunos productos y que algunos no se pueden enviar a destinos internacionales.
Al realizar un pedido, calcularemos las fechas de envío y entrega según la disponibilidad de los artículos y la opción de envío que elija. Dependiendo del proveedor de envío, las fechas estimadas de entrega podrían aparecer en la página de cotización de envío.
Tenga en cuenta que los gastos de envío de muchos de nuestros artículos se calculan según el peso. El peso de cada artículo se indica en su página de detalles. Para ajustarnos a las políticas de las empresas de transporte, todos los pesos se redondearán a la libra entera superior.
